
How is Viatris addressing climate change?
Climate change is impacting both environmental and human health. As a healthcare company with a global presence and as part of building resilient operations, Viatris works to reduce the effects on and of a changing climate. (See our Global Climate Change Policy).
We have set near-term companywide emission reduction targets in line with the latest climate science. These reduction targets have been validated and approved by the Science Based Targets Initiative (SBTi).

The SBTi classified Viatris’ scope 1 and 2 target ambition and has determined that it is aligned with the Paris Agreement’s goal of limiting global warming to 1.5°C above pre-industrial levels.
Our sites have set various short-term companywide strategies to support Viatris’ overall commitments and goals and are in line with our Global Climate Change Policy. Operations leadership has implemented several initiatives throughout the organization to make progress on global and local targets. Key actions and strategies for making progress toward our SBTi climate targets include:
- Increasing renewable energy usage
- Implementing energy-efficiency projects
- Preventing refrigerant leaks and transitioning to greener refrigerants
- Using alternative fuels and technologies
- Leveraging infrastructure upgrades and utility replacement projects
We are implementing energy-efficiency and emissions-reduction projects and are tracking GHG emissions monthly, evaluating GHG reductions annually and re-evaluating short- and long-term emission reduction strategies as needed to achieve our targets.
For examples of work across the Viatris network, performance data and progress, please see our 2024 Sustainability Report.
In addition to the above-noted climate targets, we also have companywide water and waste goals.
In 2024, we updated our climate scenario analysis to reflect operational changes including the divestiture of our API manufacturing facilities in India. The updated analysis reconfirmed that Viatris understands its key risks and has implemented relevant plans to manage risks and opportunities related to the transition toward a low-carbon economy. These existing areas of focus include protecting and enabling stable access to water and maintaining operations during extreme weather events—both of which are relevant to building resilient operations.
Our areas of focus have been protecting and enabling stable access to water, helping protect public water resources and maintaining operations during extreme weather events. The climate scenario analysis confirmed the relevance of these activities.
Working to Reduce Emissions in our Supply Chain
Our strategy to reduce GHG emissions includes increasing reliability and transport efficiency across our supply chain and all three of our freight transportation modes—road, ocean and air. To enable the shift to ocean and road freight—which is less GHG intensive than air—we have been building more time for transportation into our processes, which hinges on good demand data and forecast planning. We have a rapid response system and have established a standard operating procedure to make ocean freight our standard mode.
In 2024, Viatris significantly focused on Mode of Transport (MOT) for intercompany shipments and deliveries to our customers and piloted a new approach at our distribution center in Europe. As a result, our European operations achieved 57% of shipments by air and 43% by sea freight, marking an increase of more than 300% by sea over the previous year. Looking ahead to 2025, we plan to extend this MOT model to other Viatris sites globally. Our goal is to continue transitioning from air freight to sea freight wherever feasible throughout the year.
Engaging with Our External Suppliers
In 2024, we progressed on our supplier engagement program regarding Viatris’ target to reduce scope 3 GHG emissions by 25% by 2030, from a 2020 baseline by initiating a supplier engagement survey with key suppliers.
We actively collaborate with our suppliers to enhance the resiliency of our entire supply chain. As a full member of the Pharmaceutical Supply Chain Initiative (PSCI), we are working together to provide training and supplier engagement at scale to increase awareness across the collective supplier base on sustainable and responsible practices, with a focus on robust EHS and social risk mitigation and GHG reduction.
In addition to disclosure in our annual sustainability report, we also report to the CDP climate and water programs. Our responses are available on CDP’s public responses page and provide additional information.
How does Viatris approach water and wastewater management?
Water is a valuable natural resource, important to the health of the planet and people everywhere.
At Viatris, we work to advance responsible water stewardship in our operations and support communities’ access to clean water and sanitation. We work to understand and manage water impacts and wastewater through risk assessments, monitoring and periodic audits of all Viatris operations sites to ensure they comply with local regulatory and company water standards.
In accordance with the latest classification of water stress areas by the World Resource Institute, we have expanded our assessment program to cover our locations in Jadcherla, India; Johannesburg, South Africa; San Antonio, Texas, in the U.S.; and Dalian, China. These assessments are planned for 2025 and 2026.Moving ahead with our initiatives to reduce freshwater intake and maximize recyclability, two new zero-liquid discharge (ZLD) facilities, which eliminate wastewater discharge, are in advanced stages of implementation at Aurangabad, India, and our new injectable facility at Krishnagiri, India. With these facilities, a total of eight locations in India will apply ZLD technology, eliminating liquid discharge from the facility.
Our Water Goal: Perform water risk assessments for all locations in high or extremely high water stress areas as identified by the World Resource Institute and identify appropriate water conservation initiatives by 2025.*
Our Progress: We completed a water risk assessment for our R&D facility in Bollaram, India. With this, we completed the first phase of our goal to perform water risk assessments for all 12 sites identified under high- or extremely high-water stress areas.
*Our ability to make progress on our goals depends on several factors, some of which are outside of our control.
In India in 2024, we recovered and reused about 349,000 kL of wastewater through ZLD systems for utilities operations which is about 60% of total wastewater generated and helped to reduce our freshwater footprint. Similarly, in Australia, we collected more than 1100 kL of rainwater at our Carole Park facility and reused it across the site utilities.
We are signatory of the UN Global Compact (UNGC) and the UNGC CEO Water Mandate — a platform for business leaders to address global water challenges in collaboration with the UN, governments, civil society organizations and other stakeholders.
Our teams work to identify opportunities to improve water management within our highly regulated industry. The production requirements of our operations, coupled with local regulations and infrastructure, guide the type of water and wastewater management techniques applied. From the water risk assessments, sites will develop water conservation plans that address opportunities and risks, with vertical leaders owning the goals. All operations sites are periodically audited to ensure compliance with local regulatory and internal standards.
More information is presented in Viatris’ 2024 Sustainability Report.
How does Viatris work to limit pharmaceuticals in the environment?
The primary pathways for pharmaceuticals entering the environment from human use are by normal patient excretion, improper disposal of medicine by consumers, and the use of pharmaceuticals in agriculture and livestock. A significantly smaller contribution stems from emissions resulting from the pharmaceutical manufacturing process.
While gaps remain in the scientific link between pharmaceuticals in the environment (PiE) and human health risks, we are committed to reducing pharmaceuticals discharged from our manufacturing operations. Our approach to addressing and minimizing the potential impact of PiE from our own manufacturing is based on a wide range of activities and governance, including:
- Risk and Impact Evaluation
- Risk Reduction and Control
- Engagement and Policy
We are active participants in several trade association working groups with a focus on responsible effluent management and appropriate disposal of unused medicine.
Manufacturing Effluent Risk Assessments
As part of Viatris Global EHS Management System, we have a program and technical requirement dedicated to reducing pharmaceuticals in the environment from manufacturing. We conduct qualitative manufacturing effluent risk assessments to determine the appropriate level of control measures needed for manufacturing to protect the environment from releases of pharmaceutical ingredients.
We are expanding our quantitative manufacturing effluent risk assessments to other product classifications beyond previously completed antibiotic assessments. Viatris has a prioritization scheme to help drive the progression of these assessments from a high- to low-risk basis.
- Compliance with applicable company standards and regulatory requirements
- Implementation of defined sound wastewater management programs that are based on risk management and good engineering principles
- Utilizing published/industry API-specific discharge targets based on safe concentrations in the receiving surface waters (PNECs)
- Conducting manufacturing effluent risk assessments of wastewater containing API at our manufacturing locations; if a risk is identified, implement appropriate additional controls to mitigate the risk to an acceptable level
We are active participants in several trade association working groups with a focus on responsible effluent management and appropriate disposal of unused medicine. We also collaborated in the development and launch of the first environmental manufacturing standard for antibiotics.
Learn more about our commitments, work and progress in Viatris 2024 Sustainability Report.
How does Viatris work to reduce its environmental impact?
Recognizing that environmental and human health are interconnected, from the research and development and manufacturing of products to their delivery to customers, Viatris colleagues and partners work throughout the world to further advance sustainable operations and to minimize environmental impact while upholding a reliable supply of medicines.
Viatris has a holistic approach to and integrated management of environmental, health and safety (EHS). We work systematically and continuously to identify ways to protect the environment and minimize our impact through a comprehensive approach that focuses on managing our water, air emissions, waste and energy.Viatris’ companywide environmental goals are:
- Viatris Inc. commits to reduce absolute scope 1 and 2 greenhouse gas (GHG) emissions 42% by 2030 from a 2020 base year.* Viatris Inc. also commits to reduce absolute scope 3 GHG emissions covering purchased goods and services, capital goods, fuel and energy related activities, and upstream transportation and distribution 25% within the same timeframe. These near-term targets have been validated and approved by the Science Based Targets initiative (SBTi).
- Perform water risk assessments for all locations in high or extremely-high water risk areas as identified by the World Resource Institute and identify appropriate water conservation initiatives by 2025.
- Achieve a 50% increase in the number of zero landfill locations by 2030.
2020 as baseline
*The target boundary includes land-related emissions and removals from bioenergy feedstock
Our Global EHS Policies, including the Global Environmental Stewardship Policy, the Global Climate Change Policy, the Global Water Policy and the Global Health and Safety Policy, are based on Viatris’ 13 EHS Principles. The policies and principles apply to all Viatris global operations and every level of the organization. Viatris’ Technical Requirements establish global minimum operating requirements for various environmental and safety activities across all operations. Our global programs, guidelines and technical requirements cover topics including:
- Safety
- Waste management
- Wastewater management
- Incident management
- Chemical management
- Process safety
- Ozone-depleting substances and refrigerant management
- Air emissions
- Pharmaceuticals in the environment
- Energy management
- Water management
Implementing these policies, standards and requirements supports compliance with applicable regulations in the countries and locations where we operate, in addition to filling potential gaps where certain regulations may not exist and where our standards provide superior framework.
The EHS Management System (HSE) is based on a four-step cycle for continuous improvement:

Risk Management
At Viatris, we evaluate EHS risks for our colleagues, products, processes and facilities. Per company policies, the Global EHS Management System and technical requirements, each site must utilize EHS risk assessments using a formal process to analyze EHS risks and maintain continuous improvement plans. We assess risks to our network on an ongoing basis and take measures as appropriate to help ensure we can maintain a safe and stable supply of medicines.
Environmental risk management plans include mitigating climate change risks. As part of our risk mitigation efforts, we evaluate natural hazards and impacts from climate change across our operations. Also, our risk mitigation program covers management of ozone-depleting substances, refrigerants and GHG emissions, improving water management and increasing recycling efforts.
Our Policies
We maintain several policies governing our global environmental practices and commitments for own operations and the external supply chain, including:
- Viatris Environmental Stewardship Policy Summary
- Viatris Climate Change Policy
- Viatris Water Policy
- Viatris Code of Business Conduct and Ethics
- Viatris Supplier Code of Conduct
For a more comprehensive description of Viatris environmental work, commitments, management systems and performance, see Viatris 2024 Sustainability Report.
Are Viatris’ environmental health and safety management systems externally certified?
Viatris applies a best-in-class model for environmental health and safety (EHS) management. Our Global EHS Management System covers all of Viatris’ operations and includes global programs, technical requirements and guidelines.
While all sites are mandated to comply with Viatris’ companywide EHS program and standards, we apply a principled approach according to which each site seeks external certification on top of adherence to Viatris’ standards. We have received ISO Environmental Management and Health and Safety certifications at 30% of our sites, reflecting the strength of Viatris’ own EHS management system and standards. Reflective of divestitures in 2024, sites across our internal network that hold external certifications include:
- ISO 14001: 8 (India), 1 (Greater China), 1 (Middle East)
- ISO 50001: 1 (EU)
All India Manufacturing Sites ISO Certified
All our manufacturing facilities in India are certified to the ISO 14001 International Organization for Standardization (ISO) Environmental Management and the ISO 45001, a global standard for Occupational Safety and Health Management Systems that provides a focus on measuring and improving an organization’s safety impact. These certifications demonstrate Viatris’ leadership and our commitment to environmental stewardship and a safe work environment. They reflect the strength of our EHS management system and standards.
Achieving External Antibiotic Manufacturing Standards Certifications
All applicable Viatris manufacturing locations with antibiotic production have been internally assessed and adhere to AMRIA’s Antibiotic Manufacturing Standard, including meeting the PNEC (RQ<1) as calculated by mass balance.
Viatris has earned three BSI Kitemark Certifications under the AMRIA Manufacturing Standard – for two products at its Aurangabad, India, site and one product at its Troisdorf, Germany, manufacturing facility. The Antibiotic Manufacturing Standard, published by AMRIA and facilitated by BSI Standards Limited (BSI), provides clear guidance to manufacturers in the global antibiotic supply chain to ensure that their antibiotics are made responsibly, helping to minimize the risk of AMR in the environment. These certifications provide independent, third-party assurance and demonstrate that antibiotic residue emissions from solid and liquid waste streams are effectively controlled during manufacturing.
More details are provided in our 2024 Sustainability Report.
What is Viatris doing to address antimicrobial resistance (AMR)?
Antimicrobial resistance (AMR) is a significant global health challenge impacting millions of people around the world and with the potential to lead to even greater disruptions to care if not addressed. AMR threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi.
Viatris is a founding member and active board member of the AMR Industry Alliance (AMRIA). As the co-chair of the AMRIA Access working group, Viatris led the development of the AMRIA Equitable and Responsible Access Roadmap, published in February 2024, through a consultative and evidence-based process. The roadmap is a global policy tool focusing on increasing global access of people to appropriate and high-quality antibiotics. It highlights key barriers to diagnostics and antimicrobial access and outlines solutions to tackle the global barriers of regulatory issues, demand forecasting and procurement challenges.Fighting AMR via Responsible Manufacturing
We are founding member of AMRIA, an active member of its Manufacturing working group and is committed to partnering across the industry to collectively advance initiatives addressing AMR. Beyond working to provide access to a broad portfolio of antimicrobials and promoting appropriate use, Viatris is committed to responsible manufacturing. We are compliant with AMRIA’s Antibiotic Manufacturing Standard for our own operations and committed to implementing it across our external supply chain.
All applicable Viatris manufacturing locations with antibiotic production have been internally assessed and adhere to AMRIA’s Antibiotic Manufacturing Standard, including meeting the PNEC (RQ<1) as calculated by mass balance.
Viatris has earned three BSI Kitemark Certifications under the AMRIA Manufacturing Standard – for two products at its Aurangabad, India, site and one product at its Troisdorf, Germany, manufacturing facility. The Antibiotic Manufacturing Standard, published by AMRIA and facilitated by BSI Standards Limited (BSI), provides clear guidance to manufacturers in the global antibiotic supply chain to ensure that their antibiotics are made responsibly, helping to minimize the risk of AMR in the environment. These certifications provide independent, third-party assurance and demonstrate that antibiotic residue emissions from solid and liquid waste streams are effectively controlled during manufacturing.
In 2024, we continued to conduct assessments of our top antibiotic suppliers’ management and performance on the AMRIA Manufacturing Standard, in accordance with our five-year plan.
Collaborating to Address AMR
In 2024, the UN General Assembly convened a high-level meeting among heads of state to secure the highest level of political commitment to address AMR globally through the adoption by the UN General Assembly of the Political Declaration on AMR. Contributing to these ongoing political discussions, global news organization Foreign Policy, Viatris and other stakeholders co-hosted the “A World Without Antibiotics – Confronting the Global AMR Challenge” simulation. This meeting convened leaders and experts from government, industry and civil society to work through a simulation exploring solutions to avert the potentially devastating outcomes of unchecked AMR. During the event, Viatris and AMRIA called on member states to ensure universal, equitable, affordable and sustainable access to quality assured appropriate antibiotics and diagnostics as well as to prevent and address the drivers, sources and challenges of the environmental dimensions of AMR.
Promoting a Scalable Multi Stakeholder Model in the Fight Against AMR
In Sweden, Viatris is an active member and part of the steering committee of PLATINEA, a unique and important collaboration with authorities, educational institutions, the healthcare sector and the pharmaceutical industry. The group is working to promote more effective antibiotic use in clinics, secure supply and increase awareness of optimal use of antibiotics and dosing regimens. Viatris is a key member of PLATINEA’s work to increase the value of older, existing antibiotics in order to minimize the risk of AMR.
In 2024, Viatris collaborated with BBC Storyworks to raise awareness about PLATINEA (PLATform for INnovation of Existing Antibiotics), a unique multi-stakeholder collaboration in Sweden that includes academia, healthcare, public health authorities, public payers and regulators and the pharmaceutical industry to find solutions to AMR. The campaign highlighted the PLATINEA model beyond Sweden, sparking discussions within and beyond the policy and medical communities about replicating this initiative in other regions and driving global momentum in the fight against AMR.
To learn more about our work, please see Viatris 2024 Sustainability Report.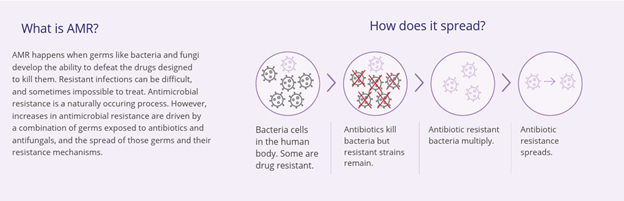
How is corporate environmental and social responsibility governed at Viatris?
Viatris’ Board of Directors oversees management’s efforts with respect to corporate environmental and social responsibility matters through its Governance and Sustainability Committee. The Global Sustainability function operates as a center of excellence within the Corporate Affairs leadership team. The Chief Corporate Affairs Officer reports directly to the CEO and communicates quarterly with the Viatris Board through the Governance and Sustainability Committee together with the Head of Global Sustainability.
On an annual basis the Governance and Sustainability Committee reviews progress with the Chief Corporate Affairs Officer on corporate environmental and social responsibility related matters that have been discussed with the Viatris Board to confirm the company is tracking its priorities in this area. The Head of Global Sustainability drives the strategic and operational development of sustainability across the company together with key partners.
The global sustainability function includes members in the U.S., Europe and India, with key partners across other functions and geographies. A multifunctional Advisory Committee comprised of global leaders with a monthly meeting cadence monitors the external landscape, company progress and supports the integration of corporate environmental and social responsibility activities across the the organization, including progress on companywide goals and priorities on access, people and the environment.
Progress on strategic focus areas and execution of relevant tasks rely on a broad and engaged network of functional leaders across the company. Additional structured forums are convened on a monthly to quarterly cadence, addressing areas of focus with regard to sustainability for specific key functions, such as the Sustainable Sourcing Council and others, complementing the advisory committee.
Learn more about our continuous work to advance more sustainable operations and responsible practices in Viatris’ 2024 Sustainability Report.
Does Viatris participate in clinical trials?
Clinical operations, including clinical trials, are key to advancing access to medicine for patients across the world. Viatris is committed to conducting clinical trials in an ethical way and promoting patient safety and the protection of patient rights throughout a study’s lifecycle.
Our clinical research program and applicable standard operating procedures are global in scope and designed to adhere to international best practice as defined in the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (CH) framework and Good Clinical Practice (GCP).
We conduct clinical trials in many regions of the world as part of the process to eventually make treatments available to patients globally. To support the further expansion of Viatris’ portfolio and bring more products to more patients with diverse needs, we are increasing the number of trials in new settings.
Global Standards for Responsible Clinical Operations
Whether our clinical trials are performed in house or by a qualified third party, the same global standards apply including adherence to GCP and promoting adherence to applicable policies, procedures and regulatory requirements. Patient safety and data integrity are at the core of our program. We develop clinical study protocols for each clinical trial that contain criteria and procedures for the conduct of every trial.
The company’s standard operating procedure governing the informed consent process is part of the quality management system. It includes detailed procedures regarding the development, review, approval, implementation and confirmation of the informed consent process for adult and pediatric trials.
Diversity in Clinical Trials
Clinical trials that include diverse populations can have better, more robust results than clinical trials that do not include diverse participants. As a global healthcare company serving 165 countries and territories, Viatris supports efforts focused on diverse representation in clinical trials and works to include varied patient populations for global studies that will be submitted for approval to health authorities around the world.
Considerations include both demographic criteria (e.g., gender, race and ethnicity) as well as non-demographic criteria (e.g., co-morbidities, organ dysfunction, the extremes of weight ranges). Viatris works with health authorities to enhance safety, scientific rigor and diverse representation in our clinical trials.
Health authorities across the globe have called for increased pediatric research to support accurate labelling for pediatric populations. Viatris is committed to complying with applicable GCP requirements to ensure pediatric clinical trial requirements are implemented with a focus on patient safety and integrity.
Required Training
All applicable colleagues and partners involved in clinical operations working on behalf of Viatris are required to be qualified by specific training, including on GCP. Further additional learning and experience are required as applicable to participate in administering clinical trials. Therapeutic area training and study-specific training are provided to applicable team members whether they are Viatris employees, partners or investigational site staff.
Risk Management in Clinical Development
The quality management system provides procedures on assessing potential risks associated with the various aspects of clinical development, such as study design, vendor selection, site selection and patient populations. The application of data analytics supports efficient trial management and oversight.
A more comprehensive description of responsible clinical operations at Viatris is presented in our 2024 Sustainability Report.
Does Viatris conduct animal testing?
We do not conduct animal testing unless it is required by national regulations. We are committed to the “3R” approach (Replacement, Reduction and Refinement) with respect to ethical animal testing. Facilities performing animal testing on our behalf are required to comply with regional scientific procedures for laboratory animal science. These facilities use and/or are approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Our Global Operations Audit (GOA) team performs regular audits on entities and facilities involved in animal testing to ensure compliance. In 2024, GOA audited eight AAALAC-certified facilities.
What is Viatris’ Access Strategy?
Access is fundamental to our mission. It is not an initiative; it is our business model. Our business and operating model is deliberately designed and implemented to deliver on our strategy to build and sustain access to medicine at scale. Underpinned by Viatris’ relevance and success in meeting evolving healthcare needs, we seek to create value for and together with our key stakeholders – the people who trust our medicines every day, the health systems who rely on us, the people who make up Viatris, our partners and the investors who believe in our ability to execute on our ambitious mission.
We are convinced that patients and systems around the world are best served by a healthcare company applying a well-rounded and long-term approach, maintaining viability while working to manage inherent risks and opportunities and continuously striving to advance sustainable operations and responsible practices in a focused way.
Access to medicine begins with sustainably delivering high-quality medicines and health solutions at scale to people, regardless of geography or circumstance. Viatris was formed to bridge the traditional divide between generics and brands, combining the best of both, to address healthcare needs more holistically globally. With an extensive portfolio of medicines to meet nearly every health need; a global, diverse and agile supply chain designed to reach more people with health solutions when and where they need them; and the scientific expertise to address some of the world’s most enduring health challenges, access is central to everything we do.
Viatris offers quality treatment options across more than 10 major therapeutic areas covering a wide variety of noncommunicable diseases (NCDs) and infectious diseases. We also enable support services such as diagnostic clinics, educational seminars and digital tools to help patients better manage their health. We offer a broad and diverse range of product options across all our therapeutic areas, with many categories containing several products in a range of dosage forms, formulations and delivery systems that allow physicians to tailor care for people’s needs.
- Served ~1 billion patients globally1
- Sold > 80 billion doses of medicine across > 165 countries and territories
- Reached ~90% of low- and lower-middle income countries
- Supplied >240 medicines on the WHO Essential Medicines List (EML), representing nearly 50% of the total list
- Supplied >135 medicines on the WHO Essential Medicines List for Children, representing >35% of the total list
- Provided 50 products on the WHO PreQ List
- Provided products that treat the top 10 of the WHO’s leading causes of death globally
- Supplied products to 100/113 countries on the Access to Medicine Foundation
- list of Access Countries
1 The number of patients served is an estimate calculated using internal sales data (global volume of doses sold in 2024 in all markets as aligned with IQVIA standard units), divided by estimated per patient usage, which is based on treatment dose, treatment duration, and treatment adherence as estimated by Viatris Medical Affairs based on approved label indication and instructions for use, current international guideline recommendations, and common usage in clinical practice. Patients using multiple Viatris medicines may be counted as multiple patients. Certain adjustments were applied to account for acceptable alternatives to the patient usage factors noted above and rounded to the nearest hundred million. Estimates may be subject to reassessment.
We are focused on meeting individual needs, whether with a generic medicine, an improved version of an existing medicine, or a truly novel therapeutic solution. We go beyond developing, making and distributing high-quality medicines and work to help find solutions that support resilient systems for healthcare. We have designed our global operations and supply chain to be a reliable and flexible partner for access across the world, constantly adapting to an ever-evolving landscape.
Partnerships and collaborations are critical, as are policies and strong healthcare systems that allow for healthy competitive environments. The needs are universal, and we work with an array of organizations - globally, regionally, locally, public and private - to support sustainable access to medicines at consistent quality standards.
Viatris works systematically to expand access via product registrations, we have several licensing agreements and work with international agencies on pooled and global procurement and regulatory harmonization.
Through our GLOBAL HEALTHCARE GATEWAY® we connect more people with even more products and services to advance access and health. Ultimately, we know we are stronger together, working collaboratively and relentlessly across our company and with the broader global community, in pursuit of access.
Goal: Provide ARV therapy equivalent to a total of 30 million patients, including >2 million children living with HIV/AIDS, between 2022 and the end of 2025.*
Our Progress: In 2024**, we provided treatments for ~7 million patients, including ~320,000 children living with HIV/AIDS. Since 2022, we have provided treatments for >24 million adults and children.
Goal: Impact 100 million patients via HCP education and outreach regarding prevention, diagnosis and treatment options for cardiovascular disease, diabetes, cancer and other important chronic conditions to improve outcomes through the NCD Academy by the end of 2025.***
Our Progress: More than 30,000 individuals have an NCD Academy account, an increase of more than 6,000 users in 2024 over the previous year. The total number of patients impacted since the launch of NCD Academy is ~115 million.1
*Our ability to make progress on our goal depends on several factors, some of which are outside of our control, including the existence and funding of our distribution partners.
** The remediation activities at the Indore site have impacted our ARV therapy supply in 2024. We are aiming to be back on track by the end of 2025.
***Our ability to make progress on our goals depends on several factors, some of which are outside of our control.
For more comprehensive description of our work, portfolio and reach, please see our 2024 Sustainability Report.
How does Viatris engage in public policy to support access to medicines?
Public policies are central factors in determining healthcare interventions and patient access. Viatris leverages our global experiences, scientific expertise and operations knowledge to support policymakers in identifying policies that advance access to quality medicines and build systems that sustain medicine availability while minimizing unintended consequences.
Viatris’ Global Policy Issue Briefs outline our positions on a variety of key policy topics. These resources are designed to share our expertise and experience with our global partners and stakeholders, offering a clear view into our understanding of the path ahead for advancing access for patients.
For more information, visit our Public Policy webpage.
How does Viatris consider price as part of its commitment to access?
At Viatris, we provide an exceptionally broad and diverse portfolio for patients across a range of major therapeutic areas, spanning both noncommunicable and infectious diseases. Our global portfolio includes best-in-class, iconic brand-name products as well as global key brands and generics, including branded and complex generics. Many of the medicines in our portfolio are not protected by patents and are subject to a general trend of price deflation over time.
As we participate in tender programs or public private partnerships around the globe, we evaluate the price of the generics within our portfolio based on an assessment of patients’ need, supply, demand, the cost of manufacturing and the affordability of our products, especially as it relates to the equivalent brand name drug, among other determinants. Other factors considered when pricing our branded portfolio include their value to patients, payers, and health systems.
Working to ensure that patients across all income levels have access to the medicines we offer means we must carefully evaluate the socioeconomic conditions within each market where Viatris does business while simultaneously advancing our ability to consistently provide patients with a reliable supply of the quality products they need. We work to provide holistic solutions for governments, NGOs and health systems globally, as we partner to connect more people to products and services.
Learn more how we work to provide access at scale in our 2024 Sustainability Report.
What are the core values that shape Viatris’ culture?
Viatris brings together committed and talented individuals who help make our shared work of building access at scale and improving healthcare around the world a reality. We have worked diligently to create a strong culture – what we call the Viatris Way - that prioritizes wellbeing; promotes inclusivity; offers training, learning and development opportunities; and enables high performance, positioning Viatris as an employer of choice for current and future colleagues.
Promoting Wellbeing
We recognize that we have a unique opportunity to live our mission by supporting colleagues through a suite of programs and resources that make up our global wellbeing program – Elevate. Through Elevate, we encourage colleagues to live life fully via our three principles of health, purpose and growth. Elevate offers support that helps all colleagues live healthy, happy and purpose-driven lives, spanning mental and physical needs and promoting activities that spark joy. We encourage colleagues to prioritize self-care in ways that are meaningful to them, fostering a sense of purpose in work and life.In 2024, we achieved our goal of ensuring that 100% of all colleagues globally had access to wellbeing and mental health resources through our employee assistance programs (EAP) and Unmind, a platform that provides workplace solutions designed by psychologists that help individuals proactively focus on wellbeing and mental health. By leveraging a variety of methods including talk therapy, video learning and expert segments, Unmind supports focusing on our whole selves.
Engagement and Employee Resource Groups
Viatris’ global Employee Resource Group (ERG) communities serve as an important opportunity to drive engagement, create meaningful awareness, lean into inclusive traits and enhance connections throughout our organization. These communities bring together colleagues from across geographies and different functions with common interests and varying experiences. These voluntary groups are open to all colleagues, fostering relationships and perspectives across the organization through global connections and events.

Developing Talent
We support our colleagues in their professional growth and the achievement of performance objectives through regular and ongoing performance, training, learning and development programs.
We encourage colleagues to make connections and collaborate with each other by living the Viatris Way via Our Expectations - Own It, Be Real, Stay Agile and Take Pride. We have more than 5,000 programs for colleagues to engage in training, learning and development. In 2024, Viatris colleagues completed more than 144,000 voluntary online trainings in areas such as enhancing personal productivity, project management and business communication. All colleagues are provided with resources and opportunities to enhance their job knowledge through on-the-job training and continuous learning. In addition to our onboarding and technical skills training, colleagues complete annual compliance, regulatory and safety trainings and other core skills trainings.
Embedding Inclusion
Our culture is centered around building trust to allow individuals to feel seen, heard and respected, and we are building a culture of engagement through leveraging the unique and differentiated perspectives and experiences of our colleagues all around the world. While our values are enduring, our approach is continuously evolving.
In 2024, our efforts focused on three themes:
- Leaning into inclusive traits such as active listening, curiosity and authenticity to allow individuals to feel welcomed
- Engaging in meaningful interactions to foster collaboration and innovation
- Engaging in connection and encouragement to allow individuals to feel valued and supported
Learn more about our work in Viatris’ 2024 Sustainability Report and on our career website.

