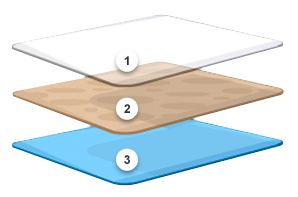
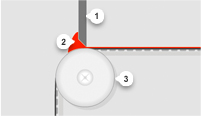
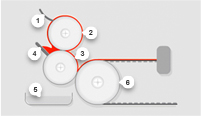
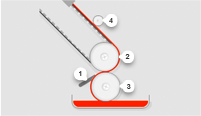
Our innovative approach leads to the design and development of exceptional thin film products. Give us your specifications or tell us what you want the material to achieve. We’ll provide custom-manufactured rolled stock made to specifications for composition, thickness, coat weight, width, pigment, and more.
Contact us today to discuss your needs at @Mtisales/STALBANS.
For job opportunities use this QR code to explore direct job listings here at MTI, or visit Viatris.com/careers.