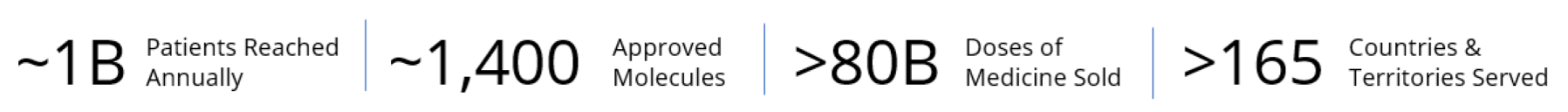
Viatris is working diligently to strengthen our position as a trusted partner in the collective efforts to improve global health and wellbeing. The world is experiencing significant challenges in the pursuit to build a more sustainable and inclusive future for everyone. As a signatory to the U.N. Global Compact, we believe companies can be important partners in the concerted work required to address many of these persistent, global challenges.
Access is at the core of our mission and our work to build more resilient healthcare systems and uphold a reliable global supply of medicines. As a global healthcare company with an exceptionally broad and diverse portfolio and global reach, our most significant contribution to society is building access to medicine and partnering for more resilient healthcare systems. However, it is not only about what we do, but also how we do it.